Analytical Quality Cycle
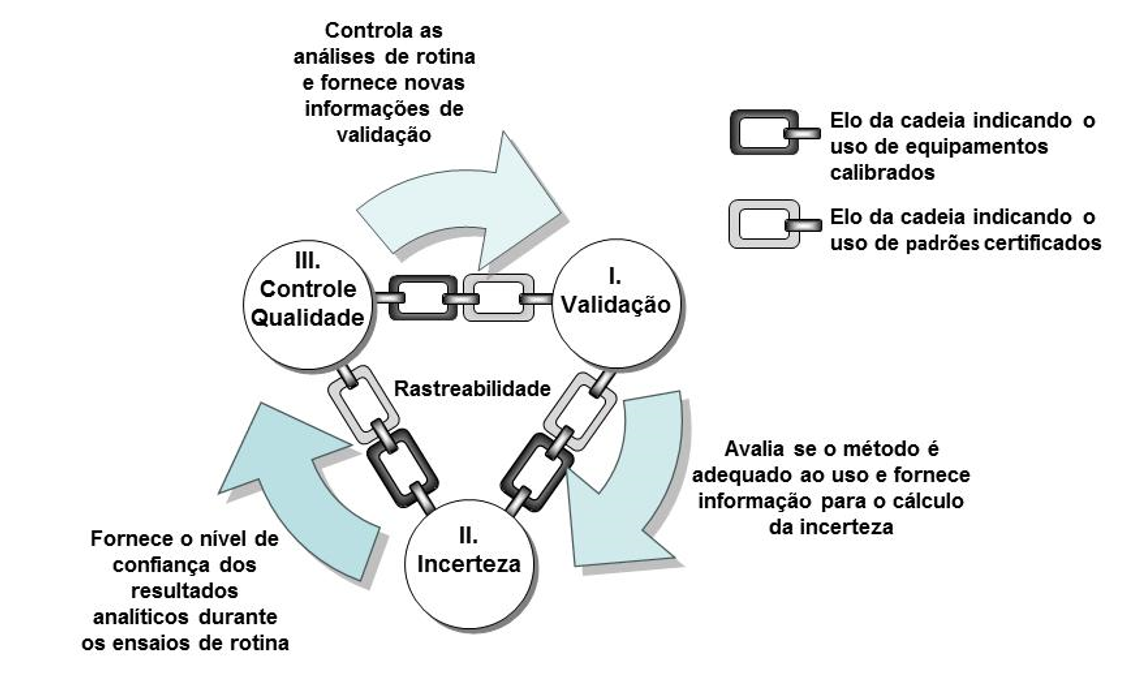
The relationship among validation, uncertainty, and quality control can be understood when implementing a new test method in a laboratory.
Initially, it is necessary to validate the method to demonstrate that it is fit for the intended use, emphasizing that during this process it is possible to learn the strengths and weaknesses of the evaluated method. Even if the method is standardized, it is always necessary to assess some validation parameter to verify whether the laboratory can obtain suitable results with the standardized method under its operating conditions (personnel, facilities, and equipment); thus, to implement a new routine test method, the first AQAC requirement—validation—must be applied.
After validation, the second step leads to the estimation of measurement uncertainty. Using a Validation-Based approach, some validation results can be used as “inputs” in the Ishikawa diagram for uncertainty calculation (for example, linearity and repeatability studies). In this way, the confidence level in the result can be determined by knowing the uncertainty value (thus applying the second AQAC requirement).
After assessing the method (during validation) and knowing the confidence level of the result (through uncertainty), the third and final step of the cycle is the application of quality control, which aims to demonstrate, during each test batch, that the method can still provide reliable results—in other words, it is a continuous validation process.
Consider that, for each sample batch, a sample of known concentration is analyzed; thus, accuracy can be evaluated immediately and, in the long term, the dispersion of a series of results can be calculated, obtaining intermediate precision. By generating new intermediate precision data (a validation parameter), uncertainty can be recalculated; hence, the AQAC keeps running like an endless loop. It is noteworthy that, to sustain the application of these requirements, the cycle is linked by “use of calibrated equipment” and “use of certified standards,” which are necessary for the reliability of the results obtained.
Considering the basic requirements to provide reliability and traceability of results, AQAC can be applied as a powerful quality tool for any type of laboratory. For laboratories with a formal quality system, such as ISO/IEC 17025 or GLP (which already addresses some AQAC requirements), its application will support and continuously improve result quality. However, AQAC also provides support for traceability, reliability, and result quality for laboratories without a formal quality system, such as research laboratories, and may be a future tool for evaluation or even accreditation for this type of laboratory [01].
References[01] OLIVARES, Igor Renato Bertoni. Quality Management in Laboratories (3rd edition – revised and expanded). 3rd ed. Campinas: Átomo e Alínea, 2015. 160 pp (3rd edition in Portuguese and English).

 VALIDATION, UNCERTAINTY AND
CONTROLE DE QUALIDADE
VALIDATION, UNCERTAINTY AND
CONTROLE DE QUALIDADE



